OCEAN ACIDIFICATION (page 67 in Moving to Higher Ground, © John Englander, 2021)
Ocean acidification is a rather obscure effect of CO2 emissions that many climate and ocean scientists believe could be the single most important change on our planet over the next century.
Much of the carbon dioxide that we put into the atmosphere dissolves in seawater as carbonic acid. You have likely tasted this fairly mild acid. It’s what gives carbonated beverages their slightly sharp, bitter taste. This increased carbonic acid lowers the pH of seawater, which affects every organism in the ocean, either directly or indirectly, through the marine food chain or habitat changes. The resulting damage can last for millennia.
As you may recall from science class, pH ranges from 1 to 14. Anything above 7 is alkaline; anything below 7 is acidic. Fresh water is neutral with a pH of 7. At the start of the fossil fuel era, ocean pH was 8.2. Now it is about 8.1. The pH scale is logarithmic, which means that a change of one integer will change the concentration tenfold, so that the movement towards acidity represents a 30 percent increase in acidity over just 240 years. The rate of change has not been this fast in hundreds of millions of years. It is 100 times faster than the last significant pH drop that occurred 650,000 years ago, and ten times faster than the most recent mass extinction event 55 million years ago[1].
Many marine organisms, from shellfish to coral reefs to plankton, depend on a calcium-rich environment to build their shells and other calcium carbonate structures. These structures simply will not form in acidic water. Figure 14 illustrates how the additional CO2 in the oceans is impeding the calcification process.
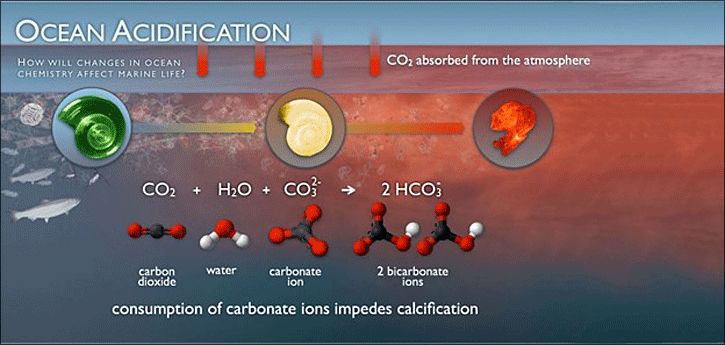
Figure 14: This graphic illustrates how carbon dioxide dissolved in seawater produces hydrogen ions that bond with carbonate ions to produce bicarbonate ions, which disrupt the calcification process in marine organisms with calcium carbonate shells or skeletons (NOAA PMEL Carbon Program
To build their shells, a carbonate ion combines with a calcium ion to form calcium carbonate. Hydrogen ions also bond with carbonate ions to produce bicarbonate. The attraction between hydrogen and carbonate is stronger than the attraction between calcium and carbonate, so the additional hydrogen ions essentially outcompete the calcium ions for the carbonate ions. In some instances, hydrogen ions can even break apart existing calcium carbonate molecules, dissolving the shells that were already formed.
This has obvious repercussions for the shell-fishing industry, but it will also affect the entire marine food web. The base of the food chain is formed by tiny marine algae called phytoplankton. They are the food of planktonic animals, which in turn are eaten by larger animals, including the biggest animal on our planet, the blue whale. The phytoplankton also produce about half of the world’s oxygen and absorb about one-third of the CO2 in the atmosphere.
Ocean acidification will significantly reduce phytoplankton growth and, in turn, leave more carbon dioxide in the atmosphere, forming a feedback loop and further raising global temperatures. Even small reductions of phytoplankton growth can have large impacts on CO2 levels.
Acidification is also extremely damaging to coral reefs. Among the most diverse ecosystems on Earth, coral reefs contain organisms from 32 of the 34 taxonomical phyla recognized by scientists. They provide shelter and habitat for millions of marine species. They also help to protect shorelines from damaging storm surges and erosion, and they play an important role in nutrient cycling. Reefs are a vital part of the recreation and tourism industry for many countries.
Corals have a relatively narrow tolerance range to changes in water pH and temperature. This is analogous to the body temperature range of humans. While we can generally tolerate a wide range of external temperatures, if our internal body temperature rises by about 5 degrees Fahrenheit (2.5 Celsius) for very long at all, we will almost certainly die. When a coral is stressed, such as from a change in temperature or pH, it will expel the colorful algae (zooxanthellae) that lives in it. This causes the coral to turn white, or “bleach.” If a reef stays bleached for long enough, it will die.
Throughout the planet’s history, the combination of ocean acidification and rising temperature have wiped out coral reefs at least five times.[2] Fossil evidence has revealed that the meteorite impact 66 million years ago that wiped out the dinosaurs, caused the pH of the ocean to drop by .25 pH units, killing three quarters of marine species.[3] This occurred in the centuries after the meteorite strike, and it took hundreds of thousands of years for carbon cycling to return to normal.
The increasing pace of reef destruction over the last few decades is unprecedented and is already affecting communities where coral reefs are central to the economy. Scientists have estimated that, at our current rate of acidification, we could see a drop of 0.4 pH units during this century, an additional 120 percent increase in acidification that will cause 70-90 percent of reefs to disappear in the next 20 years, and all of them to be gone by the end of the century.[4]
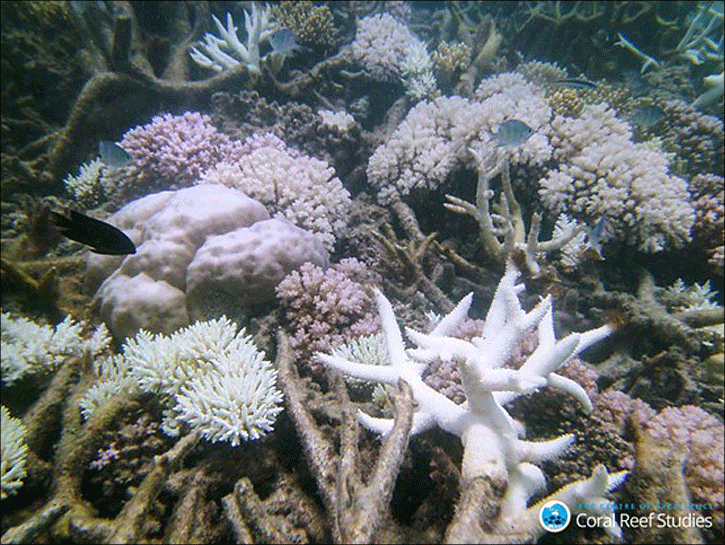
Figure 15: Bleached coral on a reef (ARC CoE for Coral Reef Studies/ Laura Richardson).
Because ocean acidification starts at the ionic level, it is hard to imagine ways to reverse the effect. There are efforts to find and develop more resilient corals that might be propagated and implanted in the ocean. But given that the fundamental chemistry of all those calcium-based shells only functions in a high alkaline environment, I am skeptical if there is a solution to the assault on the marine ecosystem, as long as we keep pumping more and more carbon dioxide into the atmosphere.
[1] John M. Guinotte and Victoria J. Fabry, “Ocean Acidification and Its Potential Effects on Marine Ecosystems”, Ann. N.Y. Acad. Sci. 1134 (2008): 320–342. doi: 10.1196/annals.1439.013
[2] Wolfgang Kiessling & Carl Simpson, “On the potential for ocean acidification to be a general cause of ancient reef crises,” Global Change Biology 17, no. 1 (December 2010): 56–67. https://doi.org/10.1111/j.1365-2486.2010.02204.
[3] Michael J. Henehan et. al. “Rapid ocean acidification and protracted Earth system recovery followed the end-Cretaceous Chicxulub impact”, PNAS 116, no. 45 (October 2019): 22500-22504, https://doi.org/10.1073/pnas.1905989116.
[4] Lauren Lipuma, “WARMING, ACIDIC OCEANS MAY NEARLY ELIMINATE CORAL REEF HABITATS BY 2100”, American Geophysical Union, February 17th, 2020, https://news.agu.org/press-release/warming-acidic-oceans-may-nearly-eliminate-coral-reef-habitats-by-2100/.